Chapter 5: Gases and Introduction to Gas Laws
5.1. Gas Pressure
Learning Objectives
By the end of this section, you will be able to:
- Define the property of pressure.
The earth’s atmosphere exerts a pressure, as does any other gas. Although we do not normally notice atmospheric pressure, we are sensitive to pressure changes—for example, when your ears “pop” during take-off and landing while flying, or when you dive underwater. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (Figure 5.1.1). Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure. In fact, normal air pressure is strong enough to crush a metal container when not balanced by equal pressure from inside the container.
A demonstration (https://www.youtube.com/watch?v=c5_ho2sc0fc) of this phenomenon is briefly explained.
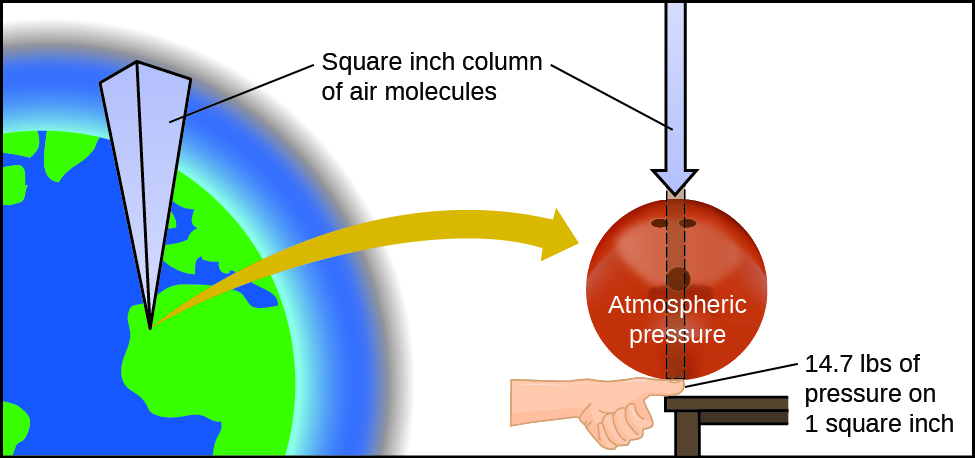
Atmospheric pressure is caused by the weight of the column of air molecules in the atmosphere above an object, such as the tanker car. At sea level, this pressure is roughly the same as that exerted by a full-grown African elephant standing on a doormat, or a typical bowling ball resting on your thumbnail. These may seem like huge amounts, and they are, but life on earth has evolved under such atmospheric pressure. If you actually perch a bowling ball on your thumbnail, the pressure experienced is twice the usual pressure, and the sensation is unpleasant.
In general, pressure is defined as the force exerted on a given area:
Note that pressure is directly proportional to force and inversely proportional to area. Thus, pressure can be increased either by increasing the amount of force or by decreasing the area over which it is applied; pressure can be decreased by decreasing the force or increasing the area.
Let’s apply this concept to determine which exerts a greater pressure in Figure 5.1.2—the elephant or the figure skater? A large African elephant can weigh 7 tons, supported on four feet, each with a diameter of about 1.5 ft (footprint area of 250 in2), so the pressure exerted by each foot is about 14 lb/in2:
The figure skater weighs about 120 lbs, supported on two skate blades, each with an area of about 2 in2, so the pressure exerted by each blade is about 30 lb/in2:
Even though the elephant is more than one hundred-times heavier than the skater, it exerts less than one-half of the pressure. On the other hand, if the skater removes their skates and stands with bare feet (or regular footwear) on the ice, the larger area over which their weight is applied greatly reduces the pressure exerted:

The SI unit of pressure is the pascal (Pa), with 1 Pa = 1 N/m2, where N is the newton, a unit of force defined as 1 kg m/s2. One pascal is a small pressure; in many cases, it is more convenient to use units of kilopascal (1 kPa = 1000 Pa) or bar (1 bar = 100,000 Pa). In the United States, pressure is often measured in pounds of force on an area of one square inch—pounds per square inch (psi)—for example, in car tires. Pressure can also be measured using the unit atmosphere (atm), which originally represented the average sea level air pressure at the approximate latitude of Paris (45°). (Table4.1) provides some information on these and a few other common units for pressure measurements
Table 5.1: Pressure units
| Pressure Units | |
|---|---|
| Unit Name and Abbreviation | Definition or Relation to Other Unit |
| pascal (Pa) | 1 Pa = 1 N/m2 recommended IUPAC unit |
| kilopascal (kPa) | 1 kPa = 1000 Pa |
| pounds per square inch (psi) | air pressure at sea level is ~14.7 psi |
| atmosphere (atm) | 1 atm = 101,325 Pa = 760 torr = 760 mm Hg air pressure at sea level is ~1 atm |
| bar (bar, or b) | 1 bar = 100,000 Pa (exactly) commonly used in meteorology |
| millibar (mbar, or mb) | 1000 mbar = 1 bar |
| inches of mercury (in. Hg) | 1 in. Hg = 3386 Pa used by aviation industry, also some weather reports |
| torr |
named after Evangelista Torricelli, inventor of the barometer |
| millimeters of mercury (mm Hg) | 1 mm Hg ~1 torr |
License and attributions:
- Chemistry: Atoms first, Second edition, 2019, Flowers, P. et al. License: CC BY 4.0. Located at https://openstax.org/books/chemistry-atoms-first-2e/pages/8-1-gas-pressure
