Chapter 12: Cellular Respiration
12.4. Oxidative Phosphorylation and Connections of other Carbohydrates, Proteins and Lipids to Pathway
Learning Objectives
By the end of this section, you will be able to:
- Describe how electrons move through the electron transport chain and explain what happens to their energy levels during this process.
- Explain how a proton (H+) gradient is established and maintained by the electron transport chain.
This is the final phase of cellular respiration and is the process that synthesizes approximately 90% of the cell’s ATP molecules. As the name implies, this phase directly uses the O2 that we inhale and it is made up of two parts: the electron transport and chemiosmosis.
Electron Transport Chain (ETC)
The ETC consists of four proteins complexes embedded into the inner membrane of mitochondria and two mobile accessory electron carriers. Electron transport is a series of redox reactions in which electrons are passed from one component to the next (Figure 12.4.1.)
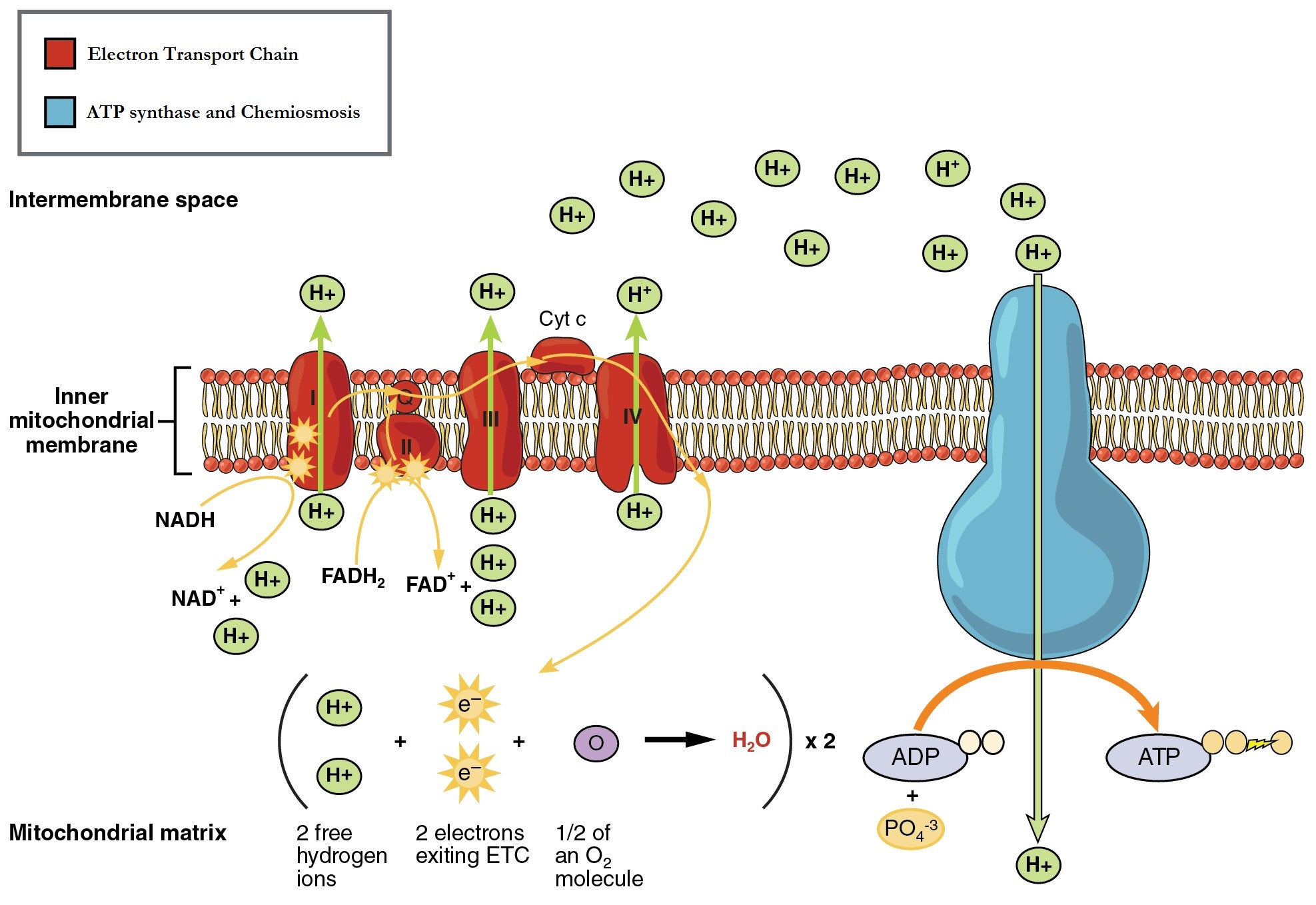
Generation of the Proton (H+) gradient
The reduced NADH and FADH2 produced during the Phases 1 and 2 of glucose catabolism provide the electrons to fuel the ETC. As these electrons are transported down the ETC, the electrons move from higher to lower energy states, an exergonic process. The energy released is used to shuttle hydrogen ions (H+) from the mitochondrial matrix, across the inner membrane, into the intermembrane space. This produces an H+ or proton gradient, and endergonic process. The energy stored in the electrochemical gradient is then used to make ATP during chemiosmosis.
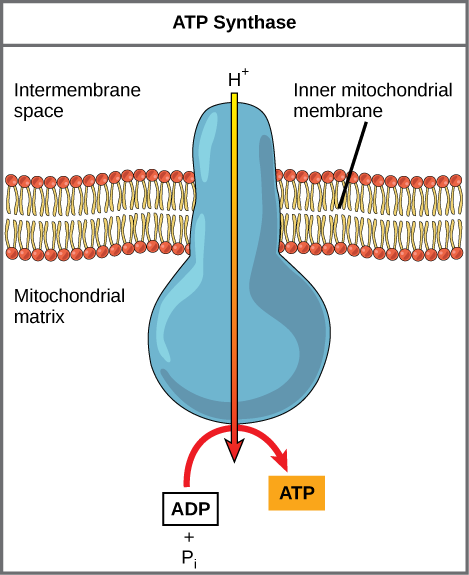
Chemiosmosis
As the ETC work, the concentration gradient of H+ ions across the membrane establishes an electrochemical gradient. The hydrogen ions’ positive charge comprises the electrical part of the gradient, and their concentration difference across the mitochondria inner membrane (higher in the intermembrane space; lower in the matrix) determines their movement ‘down’ the gradient. The H+ ions do not pass through the inner membrane bilayer, but through an integral membrane protein called ATP synthase (Figure 12.4.2.). As you saw in the biological gradients video, this complex protein acts as a tiny turbine, spun by the force of the hydrogen ions diffusing through it. The turning of this molecular machine creates mechanical energy, which facilitates the addition of a phosphate to ADP, forming ATP.
Oxygen: the final electron acceptor
Recall that pH is defined as hydrogen ions in solution. The distribution of H+ within the mitochondria is therefore called the pH gradient. Excess free H+ would be damaging to the organelle as would fee electrons. Oxygen there is indispensable for maintaining cellular homeostasis. It accepts electrons and H+ and is reduced to a nontoxic waste product, H2O. If oxygen is not present, the ETC becomes backed up and eventually shuts down. With no ETC to accept electrons from NADH and FADH2, these electron carriers remain reduced and are not available to accept additional electrons during pyruvate oxidation and the citric acid cycle. Therefore, these pathways also shut down. Most cells die in the absence of oxygen for this reason.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. The maximum yield of ATP by oxidative phosphorylation under ideal conditions is approximately 34 ATP per glucose molecule. Since four ATP are made by substrate-level phosphorylation, the total maximum yield is 38 ATP per glucose molecule.
Connections of other carbohydrates, proteins and lipids to glucose catabolism pathway
You have learned about the catabolism of glucose, which provides energy to living cells. But living things consume organic compounds other than glucose for food. How does a turkey sandwich end up as ATP in your cells? This happens because all of the catabolic pathways for carbohydrates, proteins, and lipids eventually connect into glycolysis and the citric acid cycle pathways (Figure 12.4.3.).

License and attributions:
- Biology, Second edition, 2018, Clark, M.A. et al. License: CC BY 4.0. Located at https://openstax.org/books/biology-2e/pages/7-4-oxidative-phosphorylation
- Introduction to Molecular and Cellular Biology, 2020, Mattaini, K. License: CC BY-NC 4.0. Located at https://rwu.pressbooks.pub/bio103/chapter/cellular-respiration/
- Concepts of Biology, 2013, Fowler, S. et al. License: CC BY 4.0. Located at https://openstax.org/books/concepts-biology/pages/4-3-citric-acid-cycle-and-oxidative-phosphorylation
- Biology, Second edition, 2018, Clark, M.A. et al. License: CC BY 4.0. Located at https://openstax.org/books/biology-2e/pages/7-6-connections-of-carbohydrate-protein-and-lipid-metabolic-pathways
