Chapter 3: Introductory Chemistry for Biology
3.4. Molecular Mass and the Mole Concept
Learning Objectives
By the end of this section, you will be able to:
- Calculate molecular masses for covalent and ionic compounds
- Define the amount unit mole and the related quantity Avogadro’s number
- Explain the relation between mass, moles, and numbers of atoms or molecules, and perform calculations deriving these quantities from one another
We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Dalton’s atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances.
Molecular Mass
In an earlier section, we described the development of the atomic mass unit, the concept of average atomic masses, and the use of chemical formulas to represent the elemental makeup of substances. These ideas can be extended to calculate the molecular mass of a substance by summing the average atomic masses of all the atoms represented in the substance’s molecular formula. This is also known as formula mass, formula weight and molecular weight.
Molecular Mass for Covalent Substances
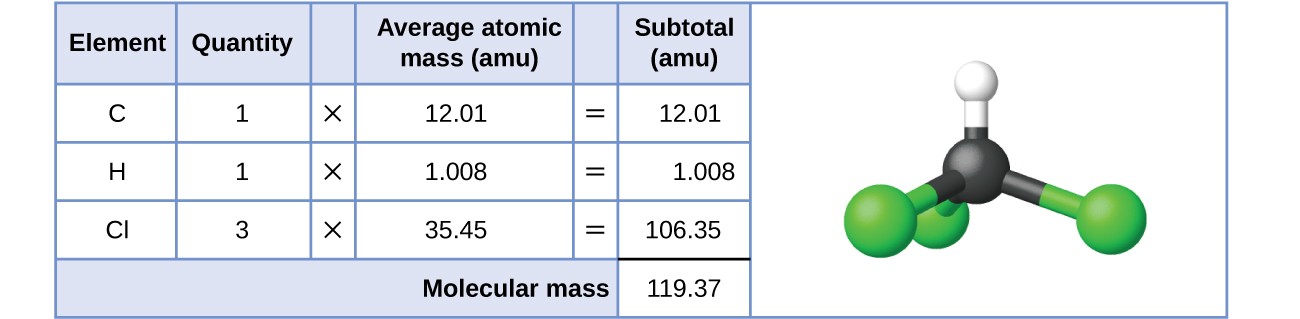
For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance. Consider chloroform (CHCl3), a covalent compound once used as a surgical anesthetic and now primarily used in the production of the “anti-stick” polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and three chlorine atoms. The average molecular mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms. Figure 3.4.1. outlines the calculations used to derive the molecular mass of chloroform, which is 119.37 AMU.
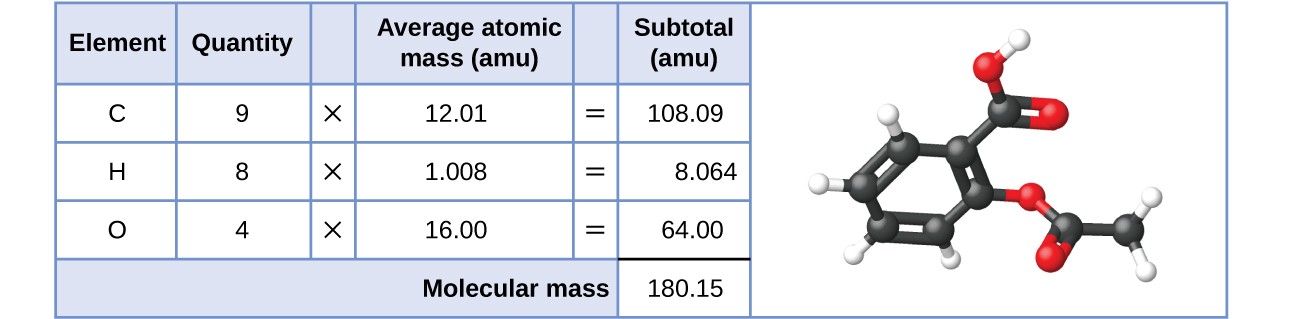
Likewise, the molecular mass of an aspirin molecule, C9H8O4, is the sum of the atomic masses of nine carbon atoms, eight hydrogen atoms, and four oxygen atoms, which amounts to 180.15 AMU (Figure 3.4.2).
Example 1: Computing Molecular Mass for a Covalent Compound
Ibuprofen, C13H18O2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Advil and Motrin. What is the molecular mass (AMU) for this compound?
Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. Following the approach described above, the average molecular mass for this compound is therefore:

Mass of Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compound’s formula.
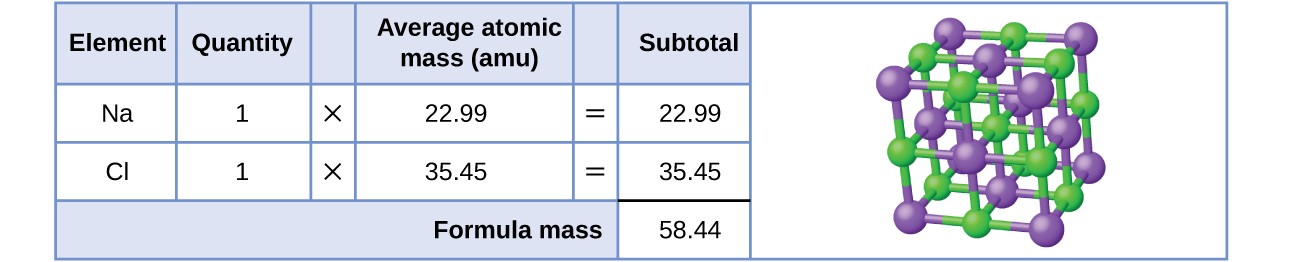
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl–, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 AMU (see Figure 3.4.4.).
Example 2: Computing Formula Mass for an Ionic Compound
Aluminum sulfate, Al2(SO4)3, is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the formula mass (AMU) of this compound?
The formula for this compound indicates it contains Al3+ and SO42- ions combined in a 2:3 ratio. For purposes of computing a formula mass, it is helpful to rewrite the formula in the simpler format, Al2S3O12. Following the approach outlined above, the molecular mass for this compound is calculated as follows:

The Mole
The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion. For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains two oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very different properties. Today, we possess sophisticated instruments that allow the direct measurement of these defining microscopic traits; however, the same traits were originally derived from the measurement of macroscopic properties (the masses and volumes of bulk quantities of matter) using relatively simple tools (balances and volumetric glassware). This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science.
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12C weighing exactly 12 g. One Latin connotation for the word “mole” is “large mass” or “bulk,” which is consistent with its use as the name for this unit. The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth.
The number of entities composing a mole has been experimentally determined to be 6.022 x 1023 ,, a fundamental constant named Avogadro’s number (NA) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of “per mole,” a conveniently rounded version being 6.022 x 1023.

Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (see Figure 3.4.6).
Because the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic or formula weight in AMU. Per the AMU definition, a single 12C atom weighs 12 AMU (its atomic mass is 12 AMU). According to the definition of the mole, 12 g of 12C contains 1 mole of 12C atoms (its molar mass is 12 g/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the AMU-reference substance, 12C. Extending this principle, the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in AMU (Figure 3.4.7.).

| Element | Average Atomic Mass (AMU) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | [latex]6.022\times {10}^{23}[/latex] |
| H | 1.008 | 1.008 | [latex]6.022\times {10}^{23}[/latex] |
| O | 16.00 | 16.00 | [latex]6.022\times {10}^{23}[/latex] |
| Na | 22.99 | 22.99 | [latex]6.022\times {10}^{23}[/latex] |
| Cl | 33.45 | 33.45 | [latex]6.022\times {10}^{23}[/latex] |

While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units (AMU versus g). To appreciate the enormity of the mole, consider a small drop of water weighing about 0.03 g (see Figure 3.4.8). The number of molecules in a single droplet of water is roughly 100 billion times greater than the number of people on earth.
Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules.
The mole is used in chemistry to represent [latex]6.022\times {10}^{23}[/latex] of something, but it can be difficult to conceptualize such a large number. Watch this video to learn more. https://www.youtube.com/watch?v=TEl4jeETVmg
The relationships between molecular mass, the mole, and Avogadro’s number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass.
Example 3: Deriving Moles from Grams for an Element
According to nutritional guidelines from the US Department of Agriculture, the estimated average requirement for dietary potassium is 4.7 g. What is the estimated average requirement of potassium in moles?
Solution:
The mass of K is provided, and the corresponding amount of K in moles is requested. Referring to the periodic table, the atomic mass of K is 39.10 AMU, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.10 g), so a reasonable “ballpark” estimate of the number of moles would be slightly greater than 0.1 mol.
The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol):

The factor-label method supports this mathematical approach since the unit “g” cancels and the answer has units of “mol:”
[latex]4.7\cancel{\text{g}}\text{K}\left(\frac{\text{mol K}}{39.10\cancel{\text{g}}}\right)=0.12\text{mol K}[/latex]
The calculated magnitude (0.12 mol K) is consistent with our ballpark expectation, since it is a bit greater than 0.1 mol.
Example 4: Deriving Grams from Moles for an Element
A liter of air contains [latex]9.2\times {10}^{-4}[/latex] mol argon. What is the mass of Ar in a liter of air?
Solution:
The molar amount of Ar is provided and must be used to derive the corresponding mass in grams. Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar, approximately 40 g. The molar amount in question is approximately one-one thousandth (~10–3) of a mole, and so the corresponding mass should be roughly one-one thousandth of the molar mass (~0.04 g):

In this case, logic dictates (and the factor-label method supports) multiplying the provided amount (mol) by the molar mass (g/mol):
[latex]9.2\times {10}^{-4}\cancel{\text{mol}}\text{Ar}\left(\frac{39.95\text{g}}{\cancel{\text{mol}}\text{Ar}}\right)=0.037\text{g Ar}[/latex]
The result is in agreement with our expectations as noted above, around 0.04 g Ar.
Example 5: Deriving Number of Atoms from Mass for an Element
Copper is commonly used to fabricate electrical wire (Figure 3.4.9.). How many copper atoms are in 5.00 g of copper wire?

Solution:
The number of Cu atoms in the wire may be conveniently derived from its mass by a two-step computation: first calculating the molar amount of Cu, and then using Avogadro’s number (NA) to convert this molar amount to number of Cu atoms:

Considering that the provided sample mass (5.00 g) is a little less than one-tenth the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth NA, or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
[latex]5.00\cancel{\text{g}}\text{Cu}\left(\frac{\cancel{\text{mol}}\text{Cu}}{63.55\cancel{\text{g}}}\right)\left(\frac{6.022\times {10}^{23}\text{atoms}}{\cancel{\text{mol}}}\right)=4.74\times {10}^{22}\text{atoms of copper}[/latex]
The factor-label method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Example 6: Deriving Moles from Grams for a Compound
Our bodies synthesize protein from amino acids. One of these amino acids is glycine, which has the molecular formula C2H5O2N. How many moles of glycine molecules are contained in 28.35 g of glycine?
Solution:
We can derive the number of moles of a compound from its mass following the same procedure we used for an element in Example 3:

The molar mass of glycine is required for this calculation, and it is computed in the same fashion as its molecular mass. One mole of glycine, C2H5O2N, contains 2 moles of carbon, 5 moles of hydrogen, 2 moles of oxygen, and 1 mole of nitrogen:

The provided mass of glycine (~28 g) is a bit more than one-third the molar mass (~75 g/mol), so we would expect the computed result to be a bit greater than one-third of a mole (~0.33 mol). Dividing the compound’s mass by its molar mass yields:
[latex]28.35\cancel{\text{g}}\text{glycine}\left(\frac{\text{mol glycine}}{75.07\cancel{\text{g}}}\right)=0.378\text{ mol glycine}[/latex]
This result is consistent with our rough estimate.
Example 7: Deriving Grams from Moles for a Compound
Vitamin C is a covalent compound with the molecular formula C6H8O6. The recommended daily dietary allowance of vitamin C for children aged 4–8 years is [latex]1.42\times {10}^{-4}\text{mol.}[/latex] What is the mass of this allowance in grams?
Solution:
As for elements, the mass of a compound can be derived from its molar amount as shown:

The molar mass for this compound is computed to be 176.124 g/mol. The given number of moles is a very small fraction of a mole (~10-4 or one-ten thousandth); therefore, we would expect the corresponding mass to be about one-ten thousandth of the molar mass (~0.02 g). Performing the calculation, we get:
[latex]1.42\times {10}^{-4}\cancel{\text{mol}}\text{vitamin C}\left(\frac{176.124\text{g}}{\cancel{\text{mol}}\text{vitamin C}}\right)=0.0250\text{g vitamin C}[/latex]
This is consistent with the anticipated result.
Example 8: Deriving the Number of Atoms and Molecules from the Mass of a Compound

A packet of an artificial sweetener contains 40.0 mg of saccharin (C7H5NO3S), which has the structural formula:
Given that saccharin has a molar mass of 183.18 g/mol, how many saccharin molecules are in a 40.0-mg (0.0400-g) sample of saccharin? How many carbon atoms are in the same sample?
Solution:
The number of molecules in a given mass of compound is computed by first deriving the number of moles, as demonstrated in Figure 5, and then multiplying by Avogadro’s number:

Using the provided mass and molar mass for saccharin yields:
[latex]0.0400\cancel{\text{g}}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S}\left(\frac{\cancel{\text{mol}}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S}}{183.18\cancel{\text{g}}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S}}\right)\left(\frac{6.022\times {10}^{23}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S molecules}}{1\cancel{\text{mol}}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S}}\right)=1.31\times {10}^{20}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S molecules}[/latex]
The compound’s formula shows that each molecule contains seven carbon atoms, and so the number of C atoms in the provided sample is:
[latex]1.31\times {10}^{20}{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S molecules}\left(\frac{7\text{C atoms}}{1{\text{C}}_{7}{\text{H}}_{5}{\text{NO}}_{3}\text{S molecule}}\right)=9.20\times {10}^{21}\text{C atoms}[/latex]
Key Concepts and Summary
The molecular mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have determined the number of entities composing 1 mole of substance to be [latex]6.022\times {10}^{23}[/latex], a quantity called Avogadro’s number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass (AMU) and molar mass (g/mol) for any substance are numerically equivalent (for example, one H2O molecule weighs approximately 18 AMU and 1 mole of H2O molecules weighs approximately 18 g).
Glossary
Avogadro’s number (NA): experimentally determined value of the number of entities comprising 1 mole of substance, equal to [latex]6.022\times {10}^{23}{\text{mol}}^{-1}[/latex]
formula mass: sum of the average masses for all atoms represented in a chemical formula; for covalent compounds, this is also the molecular mass
molar mass: mass in grams of 1 mole of a substance
mole: amount of substance containing the same number of atoms, molecules, ions, or other entities as the number of atoms in exactly 12 grams of 12C
License and attributions:
- Chemistry: Atoms first, Second edition, 2019, Flowers, P. et al. License: CC BY 4.0. Located at https://openstax.org/books/chemistry-atoms-first-2e/pages/2-4-chemical-formulas
