Chapter 9: Cell Membrane and Transport Mechanisms
9.2. Passive Transport including Osmosis
Learning Objectives
By the end of this section, you will be able to:
- Describe and contrast passive transport mechanisms of simple diffusion, facilitated diffusion and osmosis
- Define and describe tonicity in relation to water movement across the cell membrane.
- Describe cell volume changes in hyper-, hypo-, iso-tonic solutions.
Transport mechanisms
The two mechanisms for moving substances across a plasma membrane are: 1) Passive transport: the movement across the membrane without a need for chemical energy (ATP) and 2) Active transport: ATP-dependent movement across the membrane.
The concept of a concentration gradient is essential for understanding transport mechanisms. A concentration gradient is the difference in concentration of a substance across a space. Solute particles (e.g., molecules or ions) will spread/diffuse from where they are more concentrated to where they are less concentrated until they are equally distributed in that space. Movement from high to low concentration defined as moving down their concentration gradient. This directional movement is passive. No energy input is required to ‘fall down’ the gradient. In contrast energy is required to move substances from low to high concentrations, an ‘uphill’ movement against the concentration gradient.
Passive Transport
Passive transport includes the processes of diffusion (sometimes called simple diffusion), facilitated diffusion, and osmosis.
Diffusion
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration (no energy input required). An environmental example illustrates simple diffusion. Imagine being inside a closed bathroom. If a bottle of perfume were sprayed, the scent molecules would naturally diffuse from the spot where they left the bottle to all corners of the bathroom, and this diffusion would go on until no more concentration gradient remains.
Substances that diffuse across the phospholipid bilayer are nonpolar and/or relatively small. Gases (oxygen and carbon dioxide move freely across the membrane as do other small hydrophobic lipids. Water, is polar but small molecule and can diffuse across the membrane (Figure 9.2.1.).
Factors Affecting Diffusion
- Concentration gradient. The greater the difference in concentration, the more rapid the diffusion. Movement slows as substances approach equilibrium,
- Molecule size. Molecular weight and diffusion rate are inversely related. Heavier molecules diffuse more slowly; smaller molecules diffuse faster. The reverse is true for lighter molecules.
- Temperature: temperature and diffuse rate are directly related. Higher temperatures increase kinetic energy or molecular motion, increasing the diffusion rate. Low temperatures decrease diffusion rate.
- Solvent density: As the density of the solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium.
- Distance travelled: Distance and diffusion rate are inversely related. The greater the distance that a substance must travel, the slower the diffusion rate. This relates to cell shape and size. Cells in the air-blood exchange region of the lung have flattened pancake-like cells which permit rapid diffusion of oxygen and carbon dioxide gases.
Facilitated diffusion
Facilitated diffusion involves movement of a substance across the lipid bilayer with the assistance of a membrane protein. Membrane proteins facilitate movement of substances that cannot move directly across the lipid bilayer due to their size, charge, and/or polarity. Two types of membrane proteins assist these molecules: carrier proteins and channel proteins.
- Carrier proteins assist the movement of relatively large, polar substances across a cell’s membrane. There is typically a specific carrier for each molecule to be moved. The mechanism of movement involves carrier-molecule binding and protein shape changes that allow the molecule to be ‘squeezed’ through the protein core (Figure 9.2.2.). A common example is glucose, a large polar monosaccharide used by cells to make ATP. The carrier for glucose, named the glucose transporter, facilitates the inward diffusion of glucose from outside to inside the cell.
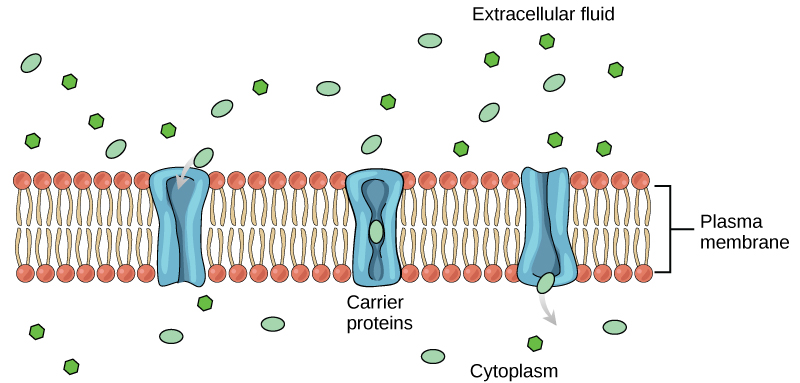
- Channel proteins have extensive hydrophilic regions allowing substances that normally would be inhibited by the lipid bilayer’s hydrophobic areas to pass across the membrane. Charged particles (electrolytes) such as Na+, K–, Cl– and polar molecules move across the membrane through channels. We said previously that small water molecules can move by simple diffusion across the membrane. Diffusion in general is a relatively slow process. There are also dedicated water channels (called aquaporins) that allow water to pass through the membrane at a very high rate. Aquaporins in the kidney play an important role in water movements.
Osmosis
Osmosis is the movement of free water molecules through a semipermeable membrane according to the water’s concentration gradient across the membrane, which is inversely proportional to the solutes’ concentration. Whereas diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. Osmosis is a special case of diffusion. Water, like other substances, moves from an area of high concentration of free water molecules to one of low free water molecule concentration. Imagine a beaker with a semipermeable membrane, separating the two sides or halves (Figure 9.2.3.). On both sides of the membrane, the water level is the same, but there are different concentrations on each side of a dissolved substance, or solute, that cannot cross the membrane. If the volume of the water is the same, but the concentrations of solute are different, then there are also different concentrations of water, the solvent, on either side of the membrane.
Tonicity
Tonicity describes how an extracellular solution can change a cell’s volume by affecting osmosis. A solution’s tonicity often directly correlates with the solution’s osmolarity. Osmolarity describes the solution’s total solute concentration. A solution with low osmolarity has a greater number of water molecules relative to the number of solute particles. A solution with high osmolarity has fewer water molecules with respect to solute particles. In a situation in which a membrane permeable to water, though not to the solute separates two different osmolarities, water will move from the membrane’s side with lower osmolarity (and more water) to the side with higher osmolarity (and less water). This effect makes sense if you remember that the solute cannot move across the membrane, and thus the only component in the system that can move—the water—moves along its own concentration gradient. An important distinction that concerns living systems is that osmolarity measures the number of particles (which may be molecules) in a solution. Therefore, a solution that is cloudy with cells may have a lower osmolarity than a solution that is clear, if the second solution contains more dissolved molecules than there are cells.
Hypotonic, Hypertonic, Isotonic Solutions
Three terms are used—hypotonic, isotonic, and hypertonic— to relate the osmolarity (solute concentration) inside the cell (intracellular) in relation to the osmolarity levels in the extracellular fluid (Figure 9.2.4.).
In a hypotonic solution The solute concentration (osmolarity) outside the cell (the extracellular fluid) is lower than the normal osmolarity inside the cells (intracellular fluid), water enters the cell by osmosis. In living systems, the point of reference is always the solute: water ratio in cytoplasm, so the prefix hypo– means that the extracellular fluid has a lower solute concentration or lower osmolarity, than the cell cytoplasm. It also means that the extracellular fluid has a higher water concentration in the solution than does the cell. In this situation, water will follow its concentration gradient and enter the cell.
In a hypertonic solution, the prefix hyper– refers to the extracellular fluid having a higher osmolarity than the cell’s cytoplasm; therefore, the fluid contains less water than the cell does. Because the cell has a relatively higher water concentration, water will leave the cell.
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the cell’s osmolarity matches that of the extracellular fluid, there will be no net movement of water into or out of the cell, although water will still move in and out. The normal sodium chloride concentration inside cells is 0.9%
Red blood cells (RBCs) are the typical model for demonstrating changes in intracellular cell volume when exposed to different tonicity solutions (Figure 9.2.4.).
License and attributions:
- Concepts of Biology, 2013, Fowler, S. et al. License: CC BY 4.0. Located at https://openstax.org/books/concepts-biology/pages/3-5-passive-transport
- Anatomy and Physiology, Second edition, 2022, Betts, J.G. et al. License: CC BY 4.0. Located at https://openstax.org/books/anatomy-and-physiology-2e/pages/3-1-the-cell-membrane
