Chapter 7: Biomolecules (Organic Molecules)
7.5. Nucleic Acids and ATP
Learning Objectives
By the end of this section, you will be able to:
- Describe nucleic acids’ structure and define the two classes of nucleic acids (DNA, RNA).
- Describe the structures and functions of DNA.
- Name the three types of RNA and describe their structure and functions.
- Describe the structures and functions of ATP.
Nucleic acids are crucial biomolecules Life: they carry the cell’s genetic blueprint for the continuity of life; they code for the synthesis of cellular proteins.
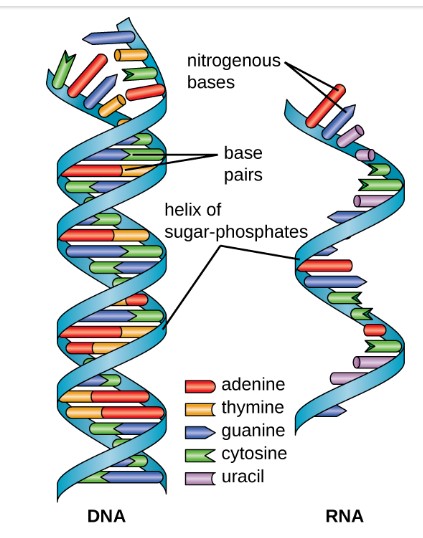
Like carbohydrates and proteins, nucleic acids form polymers. The monomer of nucleic acids are the nucleotides. A nucleotide has three parts: a phosphate group, a sugar and a nitrogenous base. The two classes of nucleic acids (DNA and RNA) vary in their sugar and nitrogenous base components.
Nucleotides of Deoxyribonucleic Acid (DNA)
The building block nucleotides that compose DNA are called deoxyribonucleotides. The three components of a deoxyribonucleotide are 1) a phosphate group, 2) a five-carbon sugar called deoxyribose, and 3) a nitrogen-containing ring structure called a nitrogenous base (Figure 7.5.1.)
The carbon atoms of DNA’s sugar are numbered 1ʹ, 2ʹ, 3ʹ, 4ʹ, and 5ʹ (1ʹ is read as “one prime”). The absence of an O atom at the 2′ position is the basis for the ‘deoxy’ in DNA’s name (Fig 7.5.1.(b))
The structure of DNA was the subject of intense study. in the 1950s. The photo below (Figure 7.5.3.) shows DNA as it appeared using a method known as X-ray crystallography. Based on the pattern shown on the X-ray, Watson and Crick hypothesized that DNA structure must be a double helix. Combined with data from other experimenters, they concluded that two nucleotide polymers were held together by purine-pyrimidine pairing, specifically A-T and C-G are the base pairs. This is the rule of complementary base pairing.
Watson & Crick published their findings on DNA structure in 1953, ending their scientific report with one of the greatest scientific understatements of all time: “It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material.” (James Watson Francis Crick. Nature, 171: 737. April 25, 1953). In fact, they had provided an explanation for how the molecule of Life replicates itself. The Nobel prize for this work was awarded in 1962 (James Watson, Francis Crick, and Maurice Wilkins).
To better understand the DNA structure, unwind the helix and imagine a ladder (Figure 7.5.4.). The sides (backbone) of the ladder are invariant, being formed by alternating sugars and phosphate groups held together by covalent bonds. The nitrogenous base pairs form the steps or rungs of the ladder. The complementary base pairs are held together by the relatively weaker hydrogen bonds (symbolized by dotted lines). Adenine (A) and thymine (T) are connected by two hydrogen bonds, and cytosine and guanine are connected by three hydrogen bonds. This organization gave the hint that it was the rungs of the DNA ladder and not the backbone that played a key role in the ability of DNA to copy itself.
The two nucleotide strands are anti-parallel; that is, one strand will have the 3′ carbon of the sugar in the “upward” position, whereas the other strand will have the 5′ carbon in the upward position. (Figure 7.5.4.)
Ribonucleic acid (RNA)
There are three classes of RNA: 1) messenger RNA, mRNA; 2) ribosomal RNA, rRNA; 3) transfer RNA, tRNA. Each class has different shapes and plays very different roles in the process of protein synthesis, a topic of future discussion. The present section focuses on the RNA’s general structural characteristics.
Like DNA, RNA is a polymer of nucleotides. Each of the nucleotides in RNA is made up of a nitrogenous base, a five-carbon sugar, and a phosphate group. Unlike DNA, the five-carbon sugar of RNA is ribose not deoxyribose. The 2′ position of RNA’s ribose contains a hydroxyl functional group (Figure 7.5.5.(a)). RNA also differs from DNA by one pyrimidine: instead of DNA’s thymine (T), RNA contains uracil (U) (Figure 7.5.5. (b)).
| Features | DNA | RNA |
|---|---|---|
| Function | Carries genetic information | Involved in protein synthesis |
| Location | Remains in the nucleus | Leaves the nucleus |
| Structure | Double helix | Usually single-stranded |
| Sugar | Deoxyribose | Ribose |
| Pyrimidines | Cytosine, thymine | Cytosine, uracil |
| Purines | Adenine, guanine | Adenine, guanine |
Adenosine Triphosphate (ATP)
ATP is a nucleotide composed of a nitrogenous base (the purine Adenine), a 5-C ribose sugar and three phosphate groups (Figure 7.5.8.). ATP is descried as the ‘energy currency’ of the cell, storing energy to be used by living cells to do ‘Work’.
Cellular work is needed in uphill processes (e.g., moving substances against a concentration gradient. That is, moving substances from areas of low to high concentrations. It’s reasonable to ask what makes the ATP molecule so special. Energy exists in many forms: ATP is chemical energy. There is also thermal energy – molecular motion that generates heat. But cells cannot used stored thermal energy to do their work because continual heat would damage and then destroy the cell. Instead, cells use the energy stored as ATP because it can be released safely on an ‘as needed’ basis.
ATP hydrolysis breaks the bond of the most unstable phosphate – the terminal (gamma) phosphate group and energy is released. The released phosphate group binds to another molecule (called phosphorylation) and raises the latter’s energy level (activates it). Kinetic energy of molecular motion then increases the probability that the activated molecule will meet another and if attracting forces are present a new chemical bond will occur. In essence, the phosphorylated (high-energy) molecule ‘dumps’ the phosphate by bonding with the new molecule. As a result, the energy level is lowered and the new molecule is stable.
In chemical terminology, ATP is an energy-coupling molecule: ATP hydrolysis (ATP > ADP + high-energy inorganic phosphate, Pi) is an energy-releasing (exergonic) reaction. This is coupled with a energy-requiring (endergonic) reaction. Without ATP, the new chemical bond formation between molecules could not occur.
License and attributions:
- Concepts of Biology, 2013, Fowler, S. et al. License: CC BY 4.0. Located at https://openstax.org/books/concepts-biology/pages/9-1-the-structure-of-dna
- Microbiology, 2016, Parker, N. et al. License: CC BY 4.0. Located at https://openstax.org/books/microbiology/pages/10-3-structure-and-function-of-rna
- Biology, Second edition, 2018, Clark, M.A. et al. License: CC BY 4.0. Located at https://openstax.org/books/biology-2e/pages/6-4-atp-adenosine-triphosphate
