Chapter 6: Introduction to Organic Chemistry
6.2. Functional Groups
Learning Objectives
By the end of this section, you will be able to:
- Describe the role of functional groups in biological molecules
Functional groups are groups of atoms that occur within molecules and confer specific chemical properties to those molecules. They are found along the “carbon backbone” or of macromolecules. This carbon backbone is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Typical atoms found in functional groups are C, H, O, N, S, and P.
Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
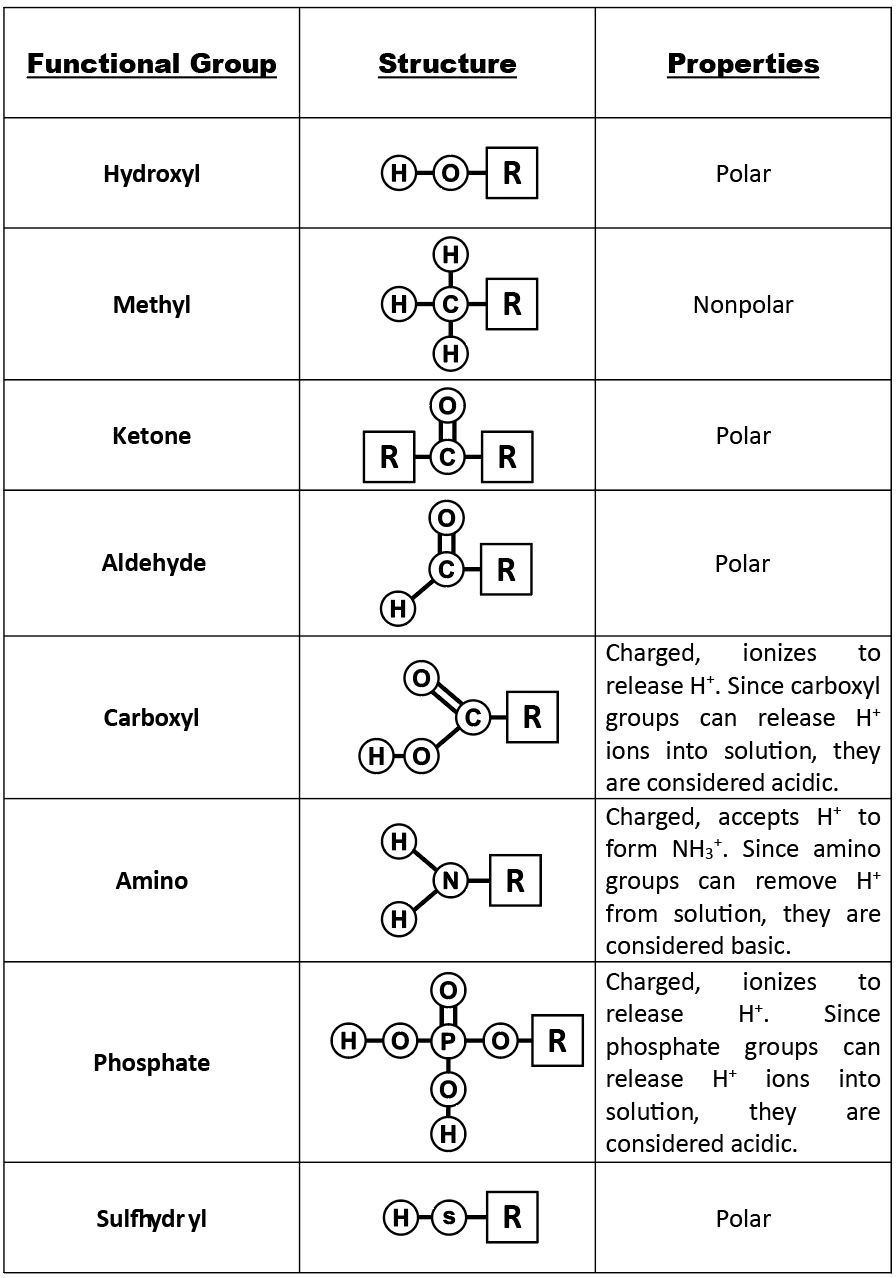
A functional group can participate in specific chemical reactions. Figure 6.2.1. shows functional groups names, their structural formulas and related chemical characteristics. Molecule shape is crucial in organic chemistry so showing how atoms are arranged gives more information about their function. To inform the reader that a functional group is part of a larger organic molecule, R- is usually added, where R indicates the remainder of the organic molecule, and the line,–, represents the covalent bond that attaches ‘R’ to the functional group by a covalent bond.
Methyl group

The methyl group is made up by a carbon atom attached to three hydrogen atoms. It is a derivative of methane, CH4.
Hydroxyl group

These are different from hydroxide ions. Bases like NaOH and KOH ionizes into OH- ion and a cation in solution. While hydroxyl groups in organic molecules do not ionizes. These molecules (R-OH) are also referred to as alcohols.
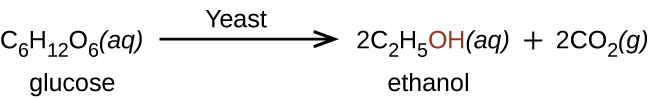
Ethanol, CH3CH2OH, also called ethyl alcohol, is a particularly important alcohol for human use. Ethanol is the alcohol produced by some species of yeast (Figure 6.2.4.) that is found in wine, beer, and distilled drinks. It has long been prepared by humans harnessing the metabolic efforts of yeasts in fermenting various sugars:
Hydroxyl groups are also found in carbohydrates and water-soluble vitamins.
Carbonyl containing functional groups
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond, commonly called a carbonyl group. The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids and esters) described in this section.
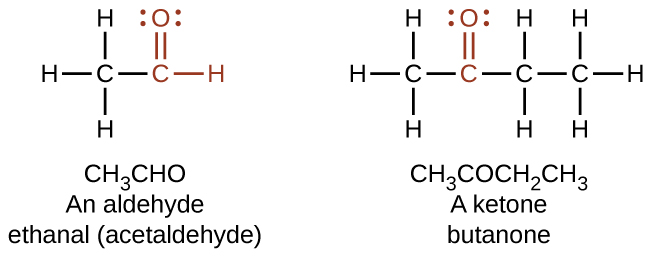
In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. In a ketone, the carbonyl group is bonded to two carbon atoms. In a carboxyl, the carbonyl is bonded to a hydroxyl group. The ester functional group resembles the carboxyl, differing only in the non-carbonyl oxygen: atom. Carboxyl’s second oxygen atom has H, forming a hydroxyl whereas ester’s second oxygen atom is covalently bonded to a carbon.
Aldehyde and ketone functional groups are the basis for distinguishing two classes of carbohydrates sugars, the aldose and ketose sugars, respectively. The carboxyl is present in the fatty acid components of the triglycerides (fats and oils) and in the amino acids of proteins. The ester bond is crucial the formation of complex nutrient lipids, the triglycerides.
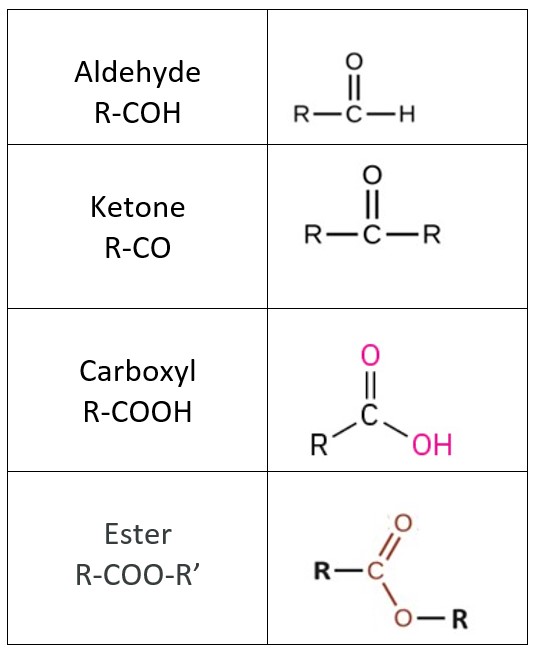
As text, an aldehyde group is represented as –CHO; a ketone is represented as –C(O)– or –CO–.
Examples
Formaldehyde, an aldehyde with the formula HCHO, is a colorless gas with a pungent and irritating odor. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by weight. Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissue to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It is also used to sterilize soil or other materials. Formaldehyde is used in the manufacture of Bakelite, a hard plastic having high chemical and electrical resistance.
Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone. It is made commercially by fermenting corn or molasses, or by oxidation of 2-propanol. Acetone is a colorless liquid. Among its many uses are as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
Organic acids contain carboxyl groups. Fatty acids, amino acids and some vitamins contain a carboxyl group. Carboxylic acids are weak acids (see the chapter on acids and bases).
Acetic acid, CH3COOH, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odor and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
Esters are produced by the reaction of acids with alcohols. The distinctive and attractive odors and flavors of many flowers, perfumes, and ripe fruits are due to the presence of one or more esters.
Connection to everyday life
For example, the ester ethyl acetate, CH3CO2CH2CH3, is formed when acetic acid reacts with ethanol (Figure 6.2.12.).
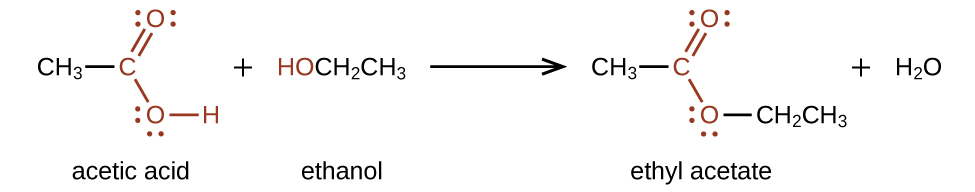
Because esters do not have hydrogen bonds between molecules, they have lower vapor pressures than the alcohols and carboxylic acids from which they are derived.
Amino group

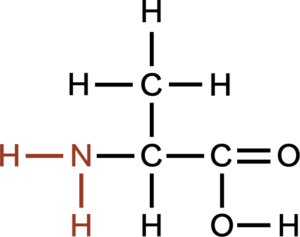
An amino group contain a nitrogen bonded to two hydrogen atoms, are able to accept H+ ions from solution, forming NH3+. Amino groups are considered basic. Amino acids, monomers of protein, and urea posse amino groups.
Phosphate group
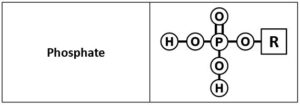
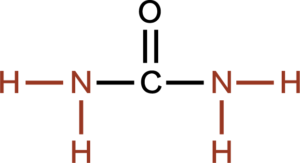
A phosphate group contains a phosphorous atom in the center with 2 hydroxyl (-OH) groups, an oxygen with a double bond and another oxygen with a single bond. The rest of the organic molecule is attached to the single bonded oxygen. Both the OH groups attached to phosphorous can lose H+ ions and are considered acidic. Phosphates are found in nucleic acids, phospholipids and high energy molecules, such as adenosine triphosphate (ATP).
A monomer of nucleic acids is a nucleotide (figure 6.2.13.(c)) and each nucleotide is made of a nitrogenous base, a pentose sugar and a phosphate group.
Section summary
The –OH group is the functional group of an alcohol. Alcohols do not ionize in water.
Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom. Carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom.
Amino groups contain a nitrogen with two hydrogens attached. They can accept protons and hence are considered bases.
Phosphate group is made of -PO2(OH)2. They can release protons and are considered acids.
Glossary
alcohol: organic compound with a hydroxyl group (–OH) bonded to a carbon atom
aldehyde: organic compound containing a carbonyl group bonded to two hydrogen atoms or a hydrogen atom and a carbon substituent
carbonyl group: carbon atom double bonded to an oxygen atom
carboxylic acid: organic compound containing a carbonyl group with an attached hydroxyl group
ester: organic compound containing a carbonyl group with an attached oxygen atom that is bonded to a carbon substituent
ketone: organic compound containing a carbonyl group with two carbon substituents attached to it
functional group: group of atoms that provides or imparts a specific function to a carbon skeleton
License and attributions:
- Biology, Second edition, 2018, Clark, M.A. et al. License: CC BY 4.0. Located at https://openstax.org/books/biology-2e/pages/2-3-carbon
- Chemistry: Atoms first, Second edition, 2019, Flowers, P. et al. License: CC BY 4.0. Located at https://openstax.org/books/chemistry-atoms-first-2e/pages/21-introduction
- Some illustrations by Lake, G., Fraley, S. License: CC BY-NC 4.0
